AOP analysis workflow
How to automate/integrate AOP analysis workflow
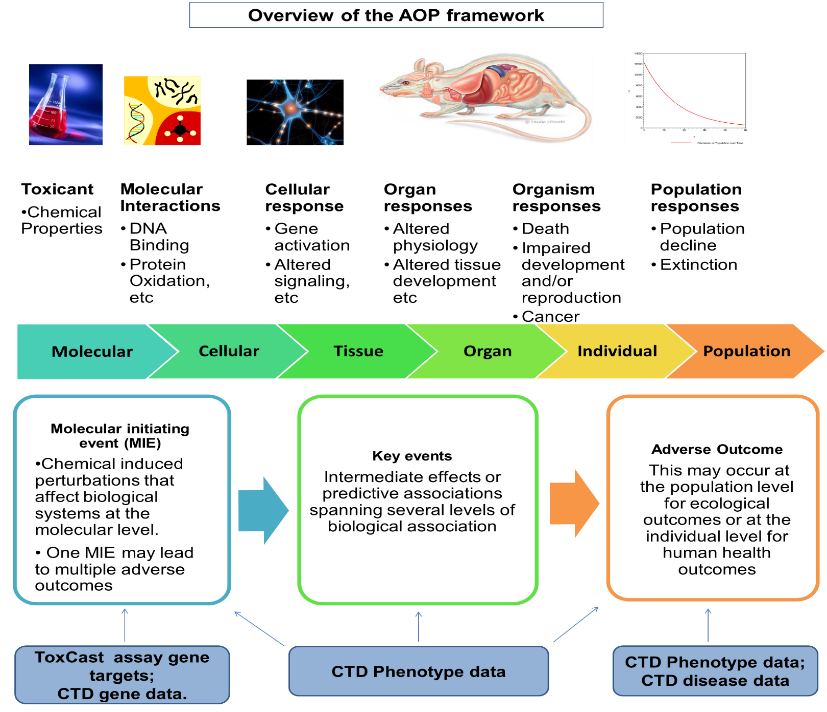 Risk assessors face increasing demands to assess more chemicals, with greater speed and accuracy, and at the same time use fewer resources and experimental animals. New approaches in biological and computational sciences are searched to be able to generate mechanistic information that could help in meeting these challenges.
Risk assessors face increasing demands to assess more chemicals, with greater speed and accuracy, and at the same time use fewer resources and experimental animals. New approaches in biological and computational sciences are searched to be able to generate mechanistic information that could help in meeting these challenges.
Edelweiss Connect Bioinformatician Dr. Noffisat O. Oki and Senior Toxicologist Dr. Tatyana Doktorova took up the challenge to present the advances made by Edelweiss Connect in the field of public data usage, analysis and integration, in a webinar hosted by the American Society for Cellular and Computational Technology (ASCCT) and European Society of Toxicology In Vitro (ESTIV). They explain how computational approaches for mechanistic data integration can be used to structure available data and make its use more effective. “Our approach builds upon a previous framework for deriving in vivo toxicity responses from in vitro data and establishes an automated workflow for Adverse Outcome Pathway (AOP) hypothesis generation,” Oki explains.
As the field of toxicology continues to evolve as a highly data-rich science, the webinar seems to have touched an important area of interest, attracting 91 participants. “Participants ranged from regulatory agencies and industry to universities all across the US and Europe,” says Tatyana. “It was nice to share what we are doing and to see such huge interest.”


