OpenRiskNet kicked off

OpenRiskNet kicked off at Technology Park in Basel, Switzerland (on 15 and 16 December 2016). All partners were present on this two-day event.
The shift from phenomenological to mechanistic toxicology and risk assessment demands for the generation of large amounts of new data based on in vitro model systems and their interpretation and extrapolation to in vivo using advanced in silico approaches. Large databases and highly sophisticated methods, algorithms and tools are becoming available to support this transition. However, their full potential cannot be used due to the fragmentation and incompatibility of the data as well as the lacking of interoperability and easy deployment of the tools. To overcome these problems, the OpenRiskNet project funded under the H2020 e-infrastructure call ““User-driven e-infrastructure innovation” was initiated to provide an infrastructure with the goal to foster general acceptance of the new alternative (non-animal) methods especially in a regulatory context. Key features are transparency and openness regarding data, workflow design and, optimally, source code, reproducibility guaranteed by openness and detailed protocolling, and standardization, harmonization, validation and quality control, which form the OpenRiskNet philosophy.
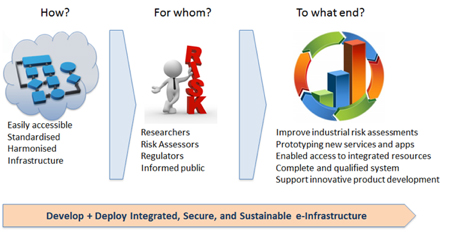
The project was launched with a two-days kick-off meeting at the Technology Park Basel on 15-16 December 2016 started with three scientific/technical presentation and then discussion meetings on the 4 work packages “WP1: Requirement Analysis, Outreach and Case Studies“, “WP2: Interoperability, Deployment and Security”, “WP3: Training, Support, Dissemination” and “WP4: Service Integration”. In these discussions it became clear that all partners agree that the only success criterion is the adoption of the infrastructure by the toxicology community. This can only be reached by integrating as many services as possible in the most accessible way and not by limiting it to the services developed by the partners. Additionally, usability testing by external users is needed during the complete development cycle. Therefore, if you are interested in providing your tool and make it interoperable with other state-of-the-art databases and analysis, modeling and visualization services or if you would like to become an early user of the infrastructure please contact us regarding the OpenRiskNet associated partner programme.
| Dr Thomas Exner is a Chief Scientific Officer (CSO) at Edelweiss Connect |


